|
GR9677 #40
|
|
|
|
|
Alternate Solutions |
BerkeleyEric
2010-09-22 22:36:21 | I think process of elimination is fastest here. From the Bohr formula we know that the n=1 is at 4*(-13.6 eV) ~ -52 eV. So it can't be (D). And it also can't be (B) since the n=2 state wouldn't be all the way up at -6 eV.
Now remember that 470 nm falls in the visible regime, so the energy difference must be at around 1-2 eV. This immediately eliminates (C) and (E) since they must be more than this amount lower than the n=4 state. |  | archard
2010-07-15 13:50:25 | You can also get it with the Rydberg formula, since you're given the wavelength of the photon and the initial energy level. Then it's just plug and chug. |  |
|
|
Comments |
cczako
2013-10-18 15:21:38 | The way I did was to just remember that E is proportional to Z^2/n^2. Its quick and easy to calculate that n=4 --> E=- 3 eV, n=3 --> E=-6 eV, n=2 --> E=-13.6 eV, and n=1 --> E=-50 eV. This automatically eliminates B and D. Then using the equation E=(hc)/lambda (I always use 1240 nm*eV for hc) you get that 470 nm is about 3 eV. So n=4 of energy -3 eV minus 3 eV gives you -6 eV. This gives you choice A. |  | BerkeleyEric
2010-09-22 22:36:21 | I think process of elimination is fastest here. From the Bohr formula we know that the n=1 is at 4*(-13.6 eV) ~ -52 eV. So it can't be (D). And it also can't be (B) since the n=2 state wouldn't be all the way up at -6 eV.
Now remember that 470 nm falls in the visible regime, so the energy difference must be at around 1-2 eV. This immediately eliminates (C) and (E) since they must be more than this amount lower than the n=4 state. |  | archard
2010-07-15 13:50:25 | You can also get it with the Rydberg formula, since you're given the wavelength of the photon and the initial energy level. Then it's just plug and chug.
sina2
2013-09-24 02:17:02 |
I tried in this way, but I failed.
|
nasim
2015-10-12 17:05:01 |
One should first have the Rydberg constant to be able to use Rydberg formula! But doesn\'t!
|
|  | asdfman
2009-11-05 00:37:16 | You can quickly narrow this down as anything less than ~400 nm is UV. If you remember that 13.6 eV, from hydrogen yields UV then that tosses out choices C, D, and E. |  | f4hy
2009-10-25 19:28:33 | I am confused. When finding 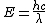 are you using the speed of light in cm but the wave length in meters? are you using the speed of light in cm but the wave length in meters?
kroner
2009-10-29 14:13:19 |
Everything is in meters there.
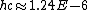 eV m or 1240 eV nm, eV m or 1240 eV nm,
which good value to know off-hand for problems like this.
|
|  | astro_allison
2005-12-08 22:50:07 | don't you mean 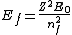 ? ? |  |
|
|
|
|
The Sidebar Chatbox...
Scroll to see it, or resize your browser to ignore it... |
|
|
|
