|
GR8677 #23
|
|
|
Problem
|
|
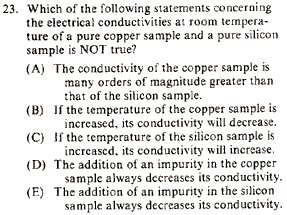 |
Advanced Topics }Solid State }Solid State
Actually, one can figure this one out with only knowledge of lower div baby physics.
(A) Electrical conductivities for conductors, semiconductors, and insulators go (in general), like this 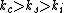 . Thus, copper should be more conductive than silicon. This is true (but one is trying to find a false statement). . Thus, copper should be more conductive than silicon. This is true (but one is trying to find a false statement).
(B) The resistivity ) , thus the conductivity, , thus the conductivity, ) . As . As 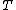 increases, increases,  decreases. This is true. decreases. This is true.
(C) Silicon is a semi-conductor, and thus it probably does not follow the same relations as (B). In fact, semiconductors have negative  temperature coefficient of resistivities. Thus, temperature coefficient of resistivities. Thus, 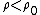 , which implies that , which implies that 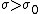 for temperature increase. for temperature increase.
(D) Doping a conductor like copper will just make it cheap. Think of cheap wire.
(E) Doping a semiconductor, however, can make it more conductive.
|
|
|
Alternate Solutions |
casseverhart13
2019-10-04 04:06:36 | yeah very nice information.... our site |  |
|
|
Comments |
casseverhart13
2019-10-04 04:06:36 | yeah very nice information.... our site |  | fredluis
2019-08-09 04:22:50 | You can eliminate almost all of the answers without really knowing anything about relativity. landscaper |  | joshuaprice153
2019-08-09 03:39:18 | This is such an amazing useful resource that you’re providing and you give it away for free. I really like seeing websites that perceive the value of providing a quality useful resource for free. Good work. towing Mt Vernon |  | jumbocrab
2010-11-12 16:11:11 | What if you added insulative impuritesto the silicon such as rubber, wouldn't that decrease its conductivity
flyboy621
2010-11-13 13:53:31 |
You have to look at words like "always" in the answers. Even if you could find some impurity that would decrease the conductivity of silicon, that still wouldn't make (E) a true statement. If there exists ANY impurity that increases the conductivity of silicon, then (E) is not true (and hence the correct choice).
|
dmn322
2017-09-24 11:44:16 |
Will even silver make copper less conductive?
|
|  | apr2010
2010-04-09 07:05:27 | (E)
pam d
2011-09-23 20:52:49 |
Nope
|
Kyle M
2012-09-19 14:02:56 |
E is the correct answer.
|
|  | thebigshow500
2008-10-13 21:53:29 | Whether you understand, just know that doping a semiconductor will push itself to be more conductive. Learn it as physicists, or the engineers are going to laugh at us! :)
garserdt216
2015-08-15 05:01:49 |
Hahaha! Glad we\'re in this together....
|
|  | bkardon
2007-10-05 20:07:42 | This makes lots of sense to me, but to be argumentative, I wonder - how can we know that doping copper with some metal, like gold, can't increase it's conductivity? Or does 'doping' specifically refer to adding some non-metallic contaminant? Just wondering.
Jeremy
2007-10-08 17:40:47 |
This is actually a really good point/question. As I understand, an impurity is any atom or molecule (regardless of conductivity) added to a pure substance. I think the key point might be that only trace amounts are added. While the new atoms/molecules may still contribute free electrons, they change the local crystal structure, and this is probably seen as an obstacle from the perspective of the free electrons. That's my guess, but I am also interested in a solid answer.
|
dean
2008-10-09 21:24:27 |
Copper conducts well because the fermi level is smackdab in the middle of a band, meaning there is essentially no energy cost to pop electrons out of their states so they can scoot around in the metal.
Semiconductors have a gap in between the valence band and the conduction band, and the fermi level lands somewhere in between. There are a few holes in the valence band and a few electrons in the conduction band, but not enough to make an electron gas.
Doping, if I understand correctly, drops a few levels in the band gap, so that it is easier to change energy level, leading to better conduction.
Doping copper should result in a few extra levels in the middle of a band. This might add a little to the conductivity, but shouldn't be nearly as effective as doping a semiconductor. Apparently there are other reasons for doping copper, but I'm not very familiar with them.
As a sidenote, doping copper with gold shouldn't do much at all, they are in the same column.
|
Ami
2009-10-11 08:33:36 |
I believe Jeremy ans was better. In the copper there is no significant gain in number of conductive electron but decrease in their sweeping velocity because of the impurities seen as obstacles.
|
Ami
2009-10-11 11:13:14 |
Sorry I meant drift velocity.
|
|  |
|
|
|
|
The Sidebar Chatbox...
Scroll to see it, or resize your browser to ignore it... |
|
|
|
