Ryry013
2019-09-07 03:00:37 | I fell for this trap by thinking of killing ants with a magnifying glass. But here\'s the difference in my opinion (correct me if I\'m wrong). When the oven normally emits radiation from it\'s 600° interior, it goes in many directions and not all the radiation hits our target object, so maybe it only reaches 100°C. Then we put a magnifying glass and, oh my! It\'s all reaching our target object. All our 600° radiation beams are reaching our target object. Now it\'s super 600°! But still only 600°... \r\n\r\nAnother example: just put the target object in the oven, get it too 600°. Thermal radiation is oversaturated, there\'s no way to get \"more\" energy. So if you try to put a magnifying glass inside the oven, it\'s not going to magically amplify the inside of the oven past what it already is. |  |
Giubenez
2014-10-13 09:50:20 | I'm not sure but I guess that the right answer is the #C due to a simple explanation:
If the sample is a BlackBody, it'll absorb the whole light provided by the lens. Since the Oven has a continuous spectrum (according to bb radiation), it will emit a constant flux of power to the lens. The whole power is focussed from the lens and thus absorbed from the black-sample.
If the sample is well insulated, and it's possible especially if it has a small radiating surface, it will increase his temperature until the equilibrium and thus until when the incoming flux will be balanced from the spontaneous radiation (on other wavelengths). Namely:
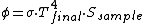
Isn't it?
Yuming Zhao
2015-10-19 09:49:52 |
I think the problem is that how can the sample be insulated at the same time when it can absorb the light by the oven.
|
|  |
Residue
2012-03-17 03:11:01 | One can assume that the light comes out of the oven with a thermal spectrum according to 600K. The lenses don't change the frequencies and hence not the spectrum and hence not the temperature of the light beam. Then it is obvious that the small object cannot be in equilibrium with the beam while maximizing the entropy. |  |
Kabuto Yakushi
2010-10-08 16:48:42 | In Thermo I learned that the transfer of energy the "wrong way" (i.e. a hot object getting hotter as a result of a cold object getting colder) does not violate any energy laws to wit: the change of energy for a system does not spontaneously increase. It just violates the second law of thermodynamics. |  |
CarlBrannen
2010-10-07 17:25:36 | Don't laugh, I actually got paid to use my metal lathe to make an optical device (visible light pipe made out of plastic) that would violate the 2nd law in the manner described by the problem. I argued and argued against it, but the engineer was insistent that I was wrong. Of course it didn't work.
SonOfHam
2010-11-12 22:45:30 |
Physicists: 1
Engineers: 0
|
NSF Fellow
2013-10-19 07:57:43 |
the best story to hear right before me taking the GRE in 1 hour.
|
|  |
alemsalem
2010-09-15 08:34:07 | suppose we had an object at some temperature and it could be described as having some particular heat capacity, and it normally absorbs radiation, and it's isolated so it can only loose heat by radiation,, where is the extra heat going??
the only good answer i can think of is that EM radiation from the oven has a different distribution for different temperatures so the oven and the sample cannot be in equilibrium, because; for example one would have a peak radiation output different than the input (hope im making sense).
|  |
shak
2010-08-20 17:19:23 | good trap:))) |  |
ramparts
2009-10-01 17:17:54 | That's naïveté. |  |
medellin
2008-10-24 17:34:29 | Why I can hot a piece of paper with a lens? You could tell me because the sun is hotter, but you cannot imagine an experiment focusing many photons of the oven so as to increase the temperature of the sample??
nobel
2008-11-04 08:41:03 |
not sure but the temperature will not increase, the heat will.
|
nobel
2008-11-04 08:42:05 |
when u talk of a cooler body, we are referring to its temperature, not how much heat it has
|
|  |
t-k-n-o
2008-06-20 19:04:16 | just consider to put the sample into de oven, its temperatue will get to 600K, but never go over 600K.!!!
evanb
2008-07-02 14:55:19 |
They're trying to lure you into computing how much power is emitted by the oven, then focus the light into a smaller area, so you have a larger power flux, then absorb that power into the sample.
Of course, we're to smart for that trap!
|
evanb
2008-07-02 14:55:47 |
"too smart", not "to smart"
|
|  |
Richard
2007-10-08 17:10:44 | I like the second solution! |  |
