|
GR9277 #77
|
|
|
|
|
Alternate Solutions |
Ning Bao
2008-01-30 07:26:00 | a common sense answer: presumably the more magnetically "efficient" (e.g. gets the same result for less material, or mass) thing would have a higher intrinsic magnetic moment. Bang for the buck theory wins again. |  |
|
|
Comments |
alemsalem
2010-09-26 03:52:11 | to see how bigger mass results in a smaller magnetic moment (classically) think of it this way:
for a given (spin) angular momentum, the one with the higher mass will have a smaller angular frequency therefore smaller current which means less magnetic moment.
this isn't a complete explanation, but it's good to have a feeling for where the mass came from. |  | Ning Bao
2008-01-30 07:26:00 | a common sense answer: presumably the more magnetically "efficient" (e.g. gets the same result for less material, or mass) thing would have a higher intrinsic magnetic moment. Bang for the buck theory wins again.
his dudeness
2010-09-05 07:02:29 |
I think this kind of reasoning could potentially get you into trouble. For example, I could say "magnetic moment, in classsical terms, is generally current times area. since a nucleus has a much larger "area" than an electron, it should have a larger magnetic moment!" which is clearly wrong.
A better way to think about this problem is to realize that particle magnetic moments are generally proportional to angular momentum, with the constant of proportionality being the "gyromagnetic ratio" (charge over mass). Since nuclei and electrons have the same charge and spin angular momentum, the only difference will be in the masses, so an electron's magnetic moment will be ~2000 times bigger.
|
|  | rmyers
2006-12-01 13:05:25 | Presumably the nucleus would also have a larger charge so the top term would increase as well. However the charge would only end up being a hundred times larger at most, while the mass is many orders of magnitude larger than an electron.
student2008
2008-10-16 08:15:53 |
You're right! Also, g-factors of electron, proton and neutron differ (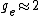 , ,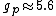 , ,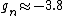 ). But this is not relevant for the ratio, for the same reason. ). But this is not relevant for the ratio, for the same reason.
|
NoPhysicist3
2017-03-23 13:24:06 |
best remark!
|
|  |
|
| Post A Comment! |
|
|
Bare Basic LaTeX Rosetta Stone
|
LaTeX syntax supported through dollar sign wrappers $, ex., $\alpha^2_0$ produces 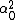 . .
|
| type this... |
to get... |
| $\int_0^\infty$ |
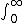 |
| $\partial$ |
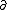 |
| $\Rightarrow$ |
 |
| $\ddot{x},\dot{x}$ |
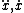 |
| $\sqrt{z}$ |
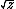 |
| $\langle my \rangle$ |
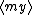 |
| $\left( abacadabra \right)_{me}$ |
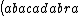_{me}) |
| $\vec{E}$ |
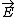 |
| $\frac{a}{b}$ |
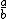 |
|
|
|
|
The Sidebar Chatbox...
Scroll to see it, or resize your browser to ignore it... |
|
|
|
