|
GR0177 #67
|
|
|
|
|
Alternate Solutions |
62a
2016-10-18 23:31:52 | You don\'t need to solve anything. Just observe that since the separated nuclei are given some energy, conservation of energy means their nucleons must now be more tightly bound. E fits, and none of the other choices is tenable. |  | QuantumCat
2014-09-22 16:44:35 | In units of MeV:
) = = 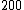
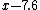 = = 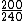
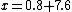
Same method as Yosun's, just a nicer look at the algebra of the problem. |  |
|
|
Comments |
62a
2016-10-18 23:31:52 | You don\'t need to solve anything. Just observe that since the separated nuclei are given some energy, conservation of energy means their nucleons must now be more tightly bound. E fits, and none of the other choices is tenable.
62a
2016-10-18 23:33:01 |
[the separated nuclei are given some *kinetic* energy, that is]
|
|  | nasim
2015-10-13 17:25:25 | Think of it like this:\r\nE(final)-E(initial)= K and E(initial)-E(final)= -K |  | QuantumCat
2014-09-22 16:44:35 | In units of MeV:
) = = 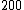
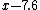 = = 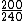
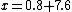
Same method as Yosun's, just a nicer look at the algebra of the problem. |  | jw111
2008-11-05 22:30:49 | binding energy is negative like David said.
so
Uf+K=Ui
240X+200=-7.6*240
X = -8.5
==========================
at beggining (set c = 1 ), the total energy is
Ei = Eo + Eb = Mi
Eb -> mass of binding energy
Eo -> rest mass energy
after fission
Ef = Eo + eb = Mf < Mi
(since Eb = -7.6*240 and eb = -8.5*240)
So the "missing mass" becomes the K |  | Poop Loops
2008-11-01 20:18:31 | Binding energy goes down as atomic number goes up, sort of like with electrons.
Therefore it wouldn't make sense for A = 120 to have *less* binding energy than A = 240.
Secondly, if you check 7.6 * 119 it is about 96MeV, therefore each fragment has to be *less* massive than half the original mass, because it states that their kinetic energy is now over 100MeV.
(A) I ruled out just because it seems weird. Also, Uranium does fission spontaneously, that's why it's used in reactors and bombs, etc.
(B ) I ruled out because of the previous calculation that says the 2 new masses are smaller than the whole mass before, but you can see that it's not by much, so "large" doesn't quite cut it.
askewchan
2008-11-07 16:55:59 |
Actually, binding energy goes down only after iron or so. Binding energy is lowest in Hydrogen, rises to a maximum around iron, then it goes down.
This is why energy is released by fusion for light elements, but by the opposite (fission) for heavy elements. The ultimate endpoint of both of these processes is iron, from which you can no longer milk any binding energy.
|
askewchan
2008-11-07 16:57:57 |
There's actually a very cool plot of this at the very bottom of the article:
http://en.wikipedia.org/wiki/Binding_energy
|
|  | jesford
2008-04-05 11:27:03 | I get answer D (6.7 MeV/nucleon) by solving Yosun's equation.... how does she get 8.5? |  | michealmas
2006-12-31 10:37:21 | I don't understand Yosun's use of signs! Using +200 on RHS gives answer D, but it implies that A=120 is a less stable atom than 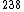 U. The famous graph of binding energy per nucleon shows that binding energy takes a maximum at Fe(the most stable atom) and decreases in both directions away from Fe. Binding energy/nucl. for A=120 must be larger than for A=238. U. The famous graph of binding energy per nucleon shows that binding energy takes a maximum at Fe(the most stable atom) and decreases in both directions away from Fe. Binding energy/nucl. for A=120 must be larger than for A=238. |  | David
2006-12-01 22:14:08 | I believe the binding energy is a form of potential energy, and must be taken to be negative.
Remember, zero binding energy means no bond and the nucleons will just fall apart. More binding energy is more stable, so systems will tend to fall into states of greater bond energy (deeper into a potential well). |  | herrphysik
2006-09-28 00:45:20 | Question: why is ) ? Shouldn't it be +100MeV? Shouldn't the initial binding energy be larger than the final binding energy since in the latter some of the energy has been converted to kinetic? Is this an error with ETS or am I missing something? ? Shouldn't it be +100MeV? Shouldn't the initial binding energy be larger than the final binding energy since in the latter some of the energy has been converted to kinetic? Is this an error with ETS or am I missing something?
travis.nicholson
2006-10-23 00:41:22 |
For clarity, it would be easier to write 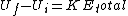 . Note that . Note that 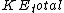 is the sum of the kinetic energies of both fragments, each of which have a is the sum of the kinetic energies of both fragments, each of which have a 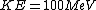 . Therefore, . Therefore, 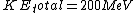
|
SonOfOle
2006-10-31 21:38:09 |
I'm with herrphysik here. With a higher binding energy per nucleon after the fission, wouldn't energy have to be added to the nuclei, not taken away in the form of KE? Anyone care to clarify this?
|
herrphysik
2006-11-02 15:06:16 |
I think the extra energy after fission comes from the difference in mass between uranium and the two fragments, and the fragments must have a higher binding energy because the uranium splits into more stable fragments.
|
mhas035
2007-04-07 20:30:47 |
Binding energy is negative, like a potential. Bigger magnitude means more negative. So a nucleon starts with it's rest mass energy then "loses" some of it when it becomes part of a nucleus. In fission the nucleons go further into the potential well (become more stable) and must release the energy they have lost (into KE in this case).
|
|  |
|
|
|
|
The Sidebar Chatbox...
Scroll to see it, or resize your browser to ignore it... |
|
|
|
