|
GR8677 #27
|
|
|
Problem
|
|
 |
Quantum Mechanics }Spin }Spin
Spin explains a lot of things.
(A) Remember orbitals? Whether a shell is full or not determines the properties of each column of the periodic table. A full shell has all electron spins paired together, while a partially filled or empty shell doesn't have that. So, spin is definitely in the Periodic Table.
(B) The specific heat of metals differs if one calculates it using the Fermi-Dirac or Bose-Einstein distributions; the first is used for fermions and the second for bosons. So, spin plays a role here.
(C) The Zeeman effect has to do with splitting caused by spin.
(D) The deflection of a moving electron is due to the magnetic field contribution to the Lorentz Force. This is a classical non-spin related phenomenon, on first analysis. This is the best choice.
(E) Fine structure has to do with splitting caused by spin.
|
|
|
Comments |
harmonxjim33
2019-10-01 14:07:00 | I believe there are many more pleasurable opportunities ahead for individuals that looked at your site. clash royale strategy |  | casseverhart13
2019-09-12 05:16:11 | Things like this are useful to solve. Gainesville Tree Pro Kensington |  | fredluis
2019-08-09 04:31:25 | Im not sure why people are talking about atomic transitions. If you want to produce x-rays in the K series, you must knock out the first electron. carpet cleaners |  | joshuaprice153
2019-08-09 02:35:29 | I think I could disagree with the main ideas. I won’t share it with my friends.. You should think of other ways to express your ideas. MacBook repair |  | QuantumCat
2014-09-02 13:43:05 | Remember the Stern-Gerlach experiment sent silver ions through an 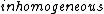 magnetic field. magnetic field. |  | VKBhartiya
2013-09-10 05:49:32 | I would say that fine structure is taking birth because of relativistic and quantum mechanical (spin) effect. This simply means that in this limit degenerate levels gets split. So now this option is not correct at all.rn |  | Manuel Abad
2012-04-04 13:57:16 | Nevertheless, there's a coupling between magnetic moment (related to spin by the gyromagnetic ratio) and the uniform magnetic field, which contributes to the hamiltonian operator. Thus, there MUST be a contribution to electron deflection coming from spin, am I wrong? If not, then ALL of the options involve spin. Then why, again, is (D) the right choice?
mpdude8
2012-04-15 20:47:38 |
I'd pick D on the basis that I learned this fact in 9th grade physics, long, long before I knew anything about spin.
C and E are both related to fine structure, and thus, I eliminate both. Both can't be right. The periodic table is broken up quite naturally by the s, p, d, and f orbitals, which are dictated by spin.
I narrowed it down to B and D. Had I not taken a stat mech course, I wouldn't have eliminated B. You can sort of "guess" D by the fact that the motion of an electron in a field is explained by high school physics.
Not a rigorous answer, but in a multiple choice environment, it really doesn't matter.
|
drizzo01
2012-10-23 12:05:52 |
To be clear, s, p, d orbitals refer to the ORBITAL angular momentum, and not the spin. That being said, the stability of these orbitals is related to whether or not they are "filled", and you can only "fill" them with spin-1/2 particles (as in, fermions). As such, spin contributes to the shape periodic table in that the placement of an element on the periodic table dictates how one might add or subtract fermions from the neutral atom's orbitals to create a more stable state (say, through chemical bonding)
|
llama
2013-10-16 19:47:02 |
The question states 'qualitatively significant' though, and the qualitatively significant factor for an electron moving in a magnetic field is simply the charge. And I'm not sure that the spin has even a small effect on the motion of a free electron in a homogeneous magnetic field - consider that the Stern-Gerlach experiment requires inhomogeneous fields.
|
|  | pam d
2011-09-23 21:23:06 | Ah, wittensdog, if only we were a part of the same pgre generation. |  | physics_gre
2009-12-24 02:47:50 | The answer given by jeka is totally wrong.Because Stern -Gerlach expt occurs in case of non uniform magnetic field. |  | jeka
2007-02-17 08:30:53 | Specific heat of metal at low temperatures is proportional to 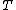 whereas specific heat of lattice is proportional to whereas specific heat of lattice is proportional to 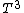 . Assume . Assume  , then heat capacity of lattice is much less than that of electron gas, which can be explained only if the electron posesses spin. , then heat capacity of lattice is much less than that of electron gas, which can be explained only if the electron posesses spin.
Anomalous Zeeman effect can be explained if there is a magnetic moment due to spin: 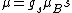
It also explaines the Stern-Gerlach effect (D).
Fine structure of atomic spectra is due to quantum-mechanical sum rule of orbital angular momentum and spin.
The first variant, (A), doesn't relate to spin because structure of the periodic table is corresponds to increase of the charge of nucleus, that is the number of protons in it.
So, the right answer is (A).
StrangeQuark
2007-07-01 12:19:53 |
Not entirely, if the periodic table is based just on the increase of the charge of nucleus then why is it not just a straight line, or why is helium not directly after hydrogen, why all the extra space between, Answer: SPIN
|
Blake7
2007-07-23 18:42:28 |
The magnetic field used in Stern-Gerlach is not uniform.
Spin gives us the shell structure of the periodic chart with features like halogens, noble gases, alkali metals and transition elements.
Yosun and StrangeQuark are correct.
|
FortranMan
2008-10-19 12:39:37 |
The  of the periodic table (or its shape if you will) corresponds to the orbital shells, s, p, d, f. Each element is grouped by its highest complete or incomplete orbital. This was first discovered empirically through chemistry by categorizing the chemical similarities and differences between different elements. If the structure of the periodic table just corresponded to the nucleus's charge (or its number of protons), than it would just be a linear list rather than the strange assortment of boxes we are used to seeing. of the periodic table (or its shape if you will) corresponds to the orbital shells, s, p, d, f. Each element is grouped by its highest complete or incomplete orbital. This was first discovered empirically through chemistry by categorizing the chemical similarities and differences between different elements. If the structure of the periodic table just corresponded to the nucleus's charge (or its number of protons), than it would just be a linear list rather than the strange assortment of boxes we are used to seeing.
|
wittensdog
2009-07-25 17:13:28 |
The Stern-Gerlach experiment does not involve moving electrons in a magnetic field; it involves neutral silver atoms with unpaired electrons. In fact, the reason that the silver atoms must be neutral is to avoid large-scale deflections due to the interaction of a moving charged particle with a magnetic field, which is indeed a very classical effect, which does not require spin for an explanation. The idea in the Stern-Gerlach experiment is that the unpaired electron results in a non-zero magnetic dipole moment, due to the electron's spin, which is why the silver atom is deflected. The way that the the electron's spin affects atomic energy levels and other aspects of atomic structure is a huge part of why the periodic table has the structure that it does. The number of valence electrons is very important for various considerations in chemistry, and the fact that the electron has spin 1/2 affects this. Also, the answers to all of these questions are provided with the released tests. These tests are checked many times by experts, and it is highly unlikely that an answer provided by ETS would be incorrect.
|
VKBhartiya
2013-09-10 05:56:20 |
I am agree with your answer, wittensdog
|
|  |
|
| Post A Comment! |
You are replying to:
Nevertheless, there's a coupling between magnetic moment (related to spin by the gyromagnetic ratio) and the uniform magnetic field, which contributes to the hamiltonian operator. Thus, there MUST be a contribution to electron deflection coming from spin, am I wrong? If not, then ALL of the options involve spin. Then why, again, is (D) the right choice?
|
Bare Basic LaTeX Rosetta Stone
|
LaTeX syntax supported through dollar sign wrappers $, ex., $\alpha^2_0$ produces 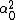 . .
|
| type this... |
to get... |
| $\int_0^\infty$ |
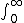 |
| $\partial$ |
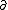 |
| $\Rightarrow$ |
 |
| $\ddot{x},\dot{x}$ |
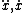 |
| $\sqrt{z}$ |
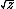 |
| $\langle my \rangle$ |
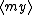 |
| $\left( abacadabra \right)_{me}$ |
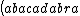_{me}) |
| $\vec{E}$ |
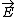 |
| $\frac{a}{b}$ |
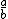 |
|
|
|
|
The Sidebar Chatbox...
Scroll to see it, or resize your browser to ignore it... |
|
|
|
