|
GR8677 #95
|
|
|
|
|
Alternate Solutions |
| There are no Alternate Solutions for this problem. Be the first to post one! |
|
|
Comments |
andrewmc
2023-07-11 19:04:29 | Can someone explain to me the solution? I don\'t get it wht E ideal gas or what is? taylordle |  | psychonautQQ
2013-09-15 16:15:45 | entropy is a state variable, therefore it is path independent. Since you end up at the same spot as you started, it must be the same. KhanAcademy thermal videos FTW :P!
|  | livieratos
2011-11-08 07:07:24 | is it right to say that since the hot reservoir is losing heat its entropy is decreasing?
nngal
2012-01-25 11:29:56 |
Yes, I think it's right. In KL the hot reservoir's entropy is decreasing while the system's entropy is increasing. In MN the system's entropy increases in the same amount so in a cycle there's no net change in the entropy of the system.
|
|  | daverigie
2009-09-21 19:37:32 | dS is an exact differential so it is zero when integrated around a closed loop |  | anmuhich
2009-03-26 09:52:40 | The whole point of a Carnot cycle is to show the theoretical maximum efficiency. By definition this means that the entropy of the system stays the same because entropy is what makes you lose efficiency. Also, this is a theoretical system, so no change in entropy is possible. |  | tera
2006-07-25 04:28:34 | In the E is indepentend of the working substance as long as it is ideal gas i think
Poop Loops
2008-11-05 23:41:43 |
No, it is always independent of the substance. The problem is you can't always get a substance to work in a Carnot cycle. But if you can, then it doesn't matter what you're using.
|
|  |
|
| Post A Comment! |
|
|
Bare Basic LaTeX Rosetta Stone
|
LaTeX syntax supported through dollar sign wrappers $, ex., $\alpha^2_0$ produces 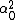 . .
|
| type this... |
to get... |
| $\int_0^\infty$ |
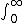 |
| $\partial$ |
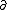 |
| $\Rightarrow$ |
 |
| $\ddot{x},\dot{x}$ |
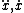 |
| $\sqrt{z}$ |
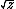 |
| $\langle my \rangle$ |
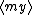 |
| $\left( abacadabra \right)_{me}$ |
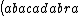_{me}) |
| $\vec{E}$ |
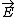 |
| $\frac{a}{b}$ |
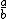 |
|
|
|
|
The Sidebar Chatbox...
Scroll to see it, or resize your browser to ignore it... |
|
|
|
