|
GR8677 #82
|
|
|
Problem
|
|
 |
Atomic }Emission Lines }Emission Lines
(A) The Stern-Gerlach effect has to do with splitting of beams of atoms sent through an inhomogenous magnetic field. Does not have to do with emission spectrum.
(B) The Stark effect has to do with energy shifts via placing atom(s) in an Electric field.
(C) Splitting is often due to electron spin magnetic moments.
(D) The condition is much too rigid.
(E) Splitting of energies means more lines than before. This choice is general enough to be true.
(The effect of energy shifts due to placing an atom in a magnetic field is called the Zeeman Effect, which isn't listed.)
|
|
|
Alternate Solutions |
| There are no Alternate Solutions for this problem. Be the first to post one! |
|
|
Comments |
mpdude8
2012-04-19 16:22:32 | Words like "always" and "never" are almost never the correct answers on problems like this. Also, it seems to me that ETS is reluctant to ask pure "definition" questions (i.e., "this effect is called what?") They're more inclined to ask you about the effect of some concept, rather than merely see if you have the name memorized. Even if I had never heard of A or B, I would be reluctant to pick them on this basis.
SurpriseAttachyon
2015-09-17 20:18:52 |
Ideally they would never do this, but that doesn\\\\\\\'t seem to be the case...
|
|  | spacemanERAU
2009-10-20 12:20:06 | I easily narrowed it down to choices C and E but how do you know to pick E instead of C...thats tricky
jcsoldier11
2009-10-25 21:55:53 |
Because as said above, the splitting is usually due to Electron spin magnetic moments, not the nuclear magnetic moments (which is what option C suggests).
|
ali8
2011-07-02 01:21:32 |
"is usually due to"....
Usually ??!!
Aren't we sure about this?
|
checkyoself
2011-10-09 14:04:51 |
The magnetic moment of an atom is much smaller than that of an electron therefore it doesn't couple to the external field as strongly. I'm assuming this is what ets want you to identify i.e. that it is 'primarily' due to the electrons, not the atoms.
|
BillNyeTheRussianSpy
2018-05-22 02:25:21 |
I just remembered 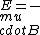 from a class where from a class where 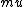 is the electron spin magnetic moment, not the nuclear magnetic moment is the electron spin magnetic moment, not the nuclear magnetic moment
|
|  | timsaucer
2006-11-01 12:43:48 | The electrons in the atoms have magnetic moment due to both their angular orbital momentum (when applicable) and their spin. When you apply the external magnetic field, it will couple with the magnetic moment either parallel or anti-parallel. The coupling will change the energy level of the electron (referred to as splitting). The splitting energy levels causes more spectral lines. |  | Healeyx76
2006-10-24 11:41:58 | Splitting of energies means more lines than before.
Why do you assume splitting of energies? All the problem states is that it differs. Why couldn't it be less?
Rune
2007-10-15 21:14:15 |
Because that is what actually happens when you put an atom in a magnetic field, known as the Zeeman effect. I don't think I've ever heard of anyone observing lines actually collapsing on eachother due to placing an atom in a magnetic field.
|
wittensdog
2009-10-07 19:24:37 |
What happens is that when you solve the SE for the coulomb potential, you get a bunch of states which all have the same energy, with different values of angular momentum. In general, the degeneracy for the nth state is n^2. However, when a magnetic field is applied, the energy also becomes a function of the angular momentum of the electrons, via their interaction with the magnetic field. So this is why there are more energy levels when the field is applied, and not less - we are lifting a degeneracy that previously existed where different angular momentum corresponded to the same energy.
|
|  |
|
| Post A Comment! |
|
|
Bare Basic LaTeX Rosetta Stone
|
LaTeX syntax supported through dollar sign wrappers $, ex., $\alpha^2_0$ produces 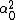 . .
|
| type this... |
to get... |
| $\int_0^\infty$ |
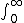 |
| $\partial$ |
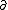 |
| $\Rightarrow$ |
 |
| $\ddot{x},\dot{x}$ |
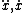 |
| $\sqrt{z}$ |
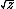 |
| $\langle my \rangle$ |
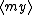 |
| $\left( abacadabra \right)_{me}$ |
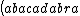_{me}) |
| $\vec{E}$ |
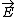 |
| $\frac{a}{b}$ |
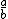 |
|
|
|
|
The Sidebar Chatbox...
Scroll to see it, or resize your browser to ignore it... |
|
|
|
