|
GR9677 #79
|
|
|
|
|
Alternate Solutions |
| There are no Alternate Solutions for this problem. Be the first to post one! |
|
|
Comments |
bluejay27
2016-12-25 22:22:27 | Check page 606 and 607. D is the correct answer.
bluejay27
2016-12-25 22:22:53 |
of Young and Freedman
|
|  | hellothar
2012-10-21 20:26:23 | No one seems to have pointed out that Cv, for a DIatomic gas, at low temperatures is not 3/2R but 5/2R. (Check HRK, or Fermi, or anywhere else).
Yosun's 3/2R applies only to monoatomic molecules. The problem asks about diatomic molecules.
hellothar
2012-10-21 20:33:06 |
Nope. Nevermind. The standard texts are assuming only 3+2 vibrational modes. For very, very low temperatures, 3/2 is right.
|
|  | shafatmubin
2009-11-04 15:17:49 | This link might help:
http://hyperphysics.phy-astr.gsu.edu/HBASE/hframe.html
It shows the variation of Cv of hydrogen gas with temperature. At very high temperatures, above 1000 K, there are 7 degrees of freedom because two additional degrees (apart from the 3 translational and 2 rotational degrees) become active. Can anyone explain what are these two new degrees of freedom? One of them is possibly vibrational, but what about the other?
hdcase
2009-11-04 21:46:12 |
Hyperphysics doesn't make this very clear, but wikipedia does:
http://en.wikipedia.org/wiki/Specific_heat_capacity#Diatomic_gas
The vibrational state (there is one degree of freedom from it, for a total of 6 degrees of freedom) adds R (instead of R/2) because it carries both a potential and kinetic term (both of which can "hold energy," which is essentially what the specific heat measures).
|
shafatmubin
2009-11-05 13:12:22 |
Thanks hdcase. Vibrational energy contributing R instead of 2R makes things a lot more understandable.
|
|  | petr1243
2008-03-18 11:45:15 | Roughly, Rotaional effects disappear below 25k, and all 3 effects will produce a specific heat of 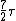 at temperatures above 8000K. A good reference is ch.18 of the University Physics text. at temperatures above 8000K. A good reference is ch.18 of the University Physics text. |  | cyberdeathreaper
2007-02-03 15:05:37 | I found this equation helpful in solving the problem:
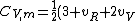R)
Where
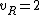
for linear molecules, and
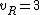
for non-linear molecules.
I assumed that that
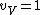
for very high temperatures. And of course, the rotational and vibrational contributions are 0 at very low temperatures, as Yosunism explained in the answer.
faith
2010-11-11 20:24:18 |
equipartition says each degree of freedom has 0.5 K.E.
translational has 3 mode of d.f
rotational
for diatomic ( linear) 2 mode
triatomic has 3x2 mode =6
polyatomic 3 mode (why ? i do not know)
vibrational (i need help completing this)
diatomic = 1 (or 2) mode?
triatomic=?
poly=?
to the question
finding the df for high tem
1/2(3+2+1?)=1?
if its vibrational has 2 mode: 1/2(3+2+2)=7/2
so the answer could be D or E. any formula for vibrational mode under high temp?
|
|  |
|
| Post A Comment! |
|
|
Bare Basic LaTeX Rosetta Stone
|
LaTeX syntax supported through dollar sign wrappers $, ex., $\alpha^2_0$ produces 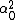 . .
|
| type this... |
to get... |
| $\int_0^\infty$ |
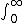 |
| $\partial$ |
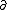 |
| $\Rightarrow$ |
 |
| $\ddot{x},\dot{x}$ |
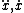 |
| $\sqrt{z}$ |
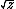 |
| $\langle my \rangle$ |
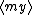 |
| $\left( abacadabra \right)_{me}$ |
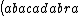_{me}) |
| $\vec{E}$ |
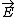 |
| $\frac{a}{b}$ |
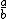 |
|
|
|
|
The Sidebar Chatbox...
Scroll to see it, or resize your browser to ignore it... |
|
|
|
